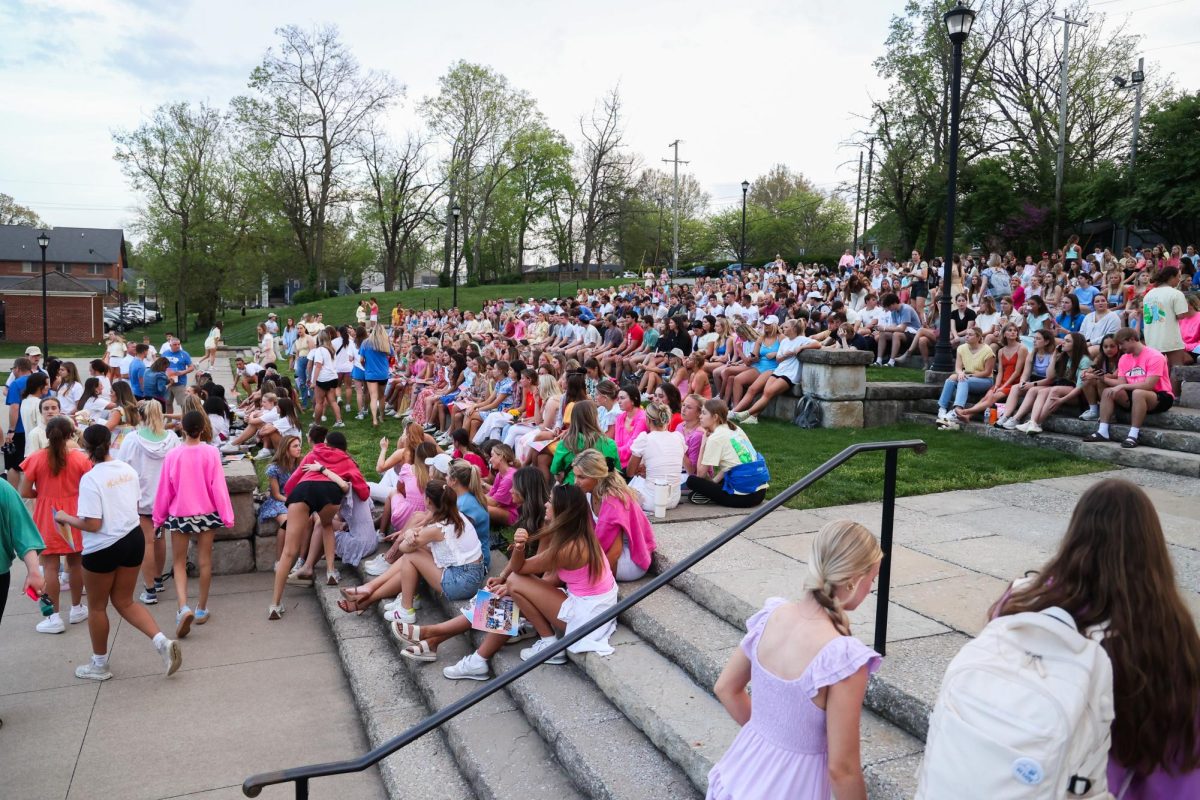UK selected to participate in COVID-19 vaccine booster study
August 11, 2021
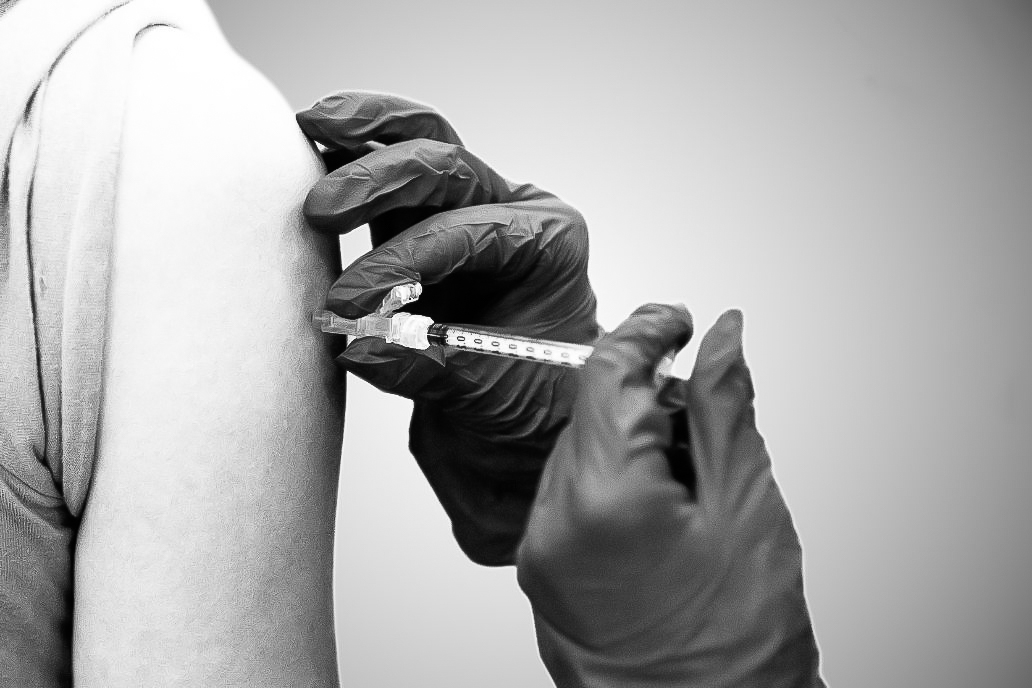
UK researchers will be part of a national study to assess a COVID-19 vaccine booster, UK announced in a media availability meeting and news release Wednesday.
Adults who have received two doses of the Pfizer vaccine by the end of February 2021 are eligible to receive doses of this Johnson & Johnson vaccine booster. Those who participated in UK’s previous trial of the Johnson & Johnson vaccine are also eligible. All participants must have never contracted COVID-19 and must be in good health.
Participants will be randomized into groups, and researchers will monitor their immune response to different regimens of the booster. Participants will also be compensated.
Dr. Richard Greenberg, an infectious disease specialist in UK HealthCare, leads this study. He said in the media availability meeting that the purpose of the booster trial is to stay ahead of COVID-19 mutations and variants.
“We don’t have the data to support boosters at the moment…what we do know is the Delta variant appears to be contagious even in vaccinated individuals,” he said. “We don’t know how long the vaccinated people can spread the virus; that needs to be researched.”
“We don’t know what’s going to happen; this is why we’re doing the study,” Dr. Edgar Hoover, who manages the study, said in the media availability meeting. “We don’t know if boosters are going to be recommended…That’s the main purpose of this booster dose trial.”
The study is currently scheduled to last six months, with the possibility of being extended.
“We are all enjoying going back to some semblance of our normal lives, but we need to know more about COVID-19 booster shots,” Dr. Philip Kern, a co-investigator in the study, said in the news release. “We’re happy to play a part in ensuring the health of Kentuckians.”
For more information or to sign up for the study, visit stopcovidky.com.