UK COVID-19 boosters no longer FDA-approved
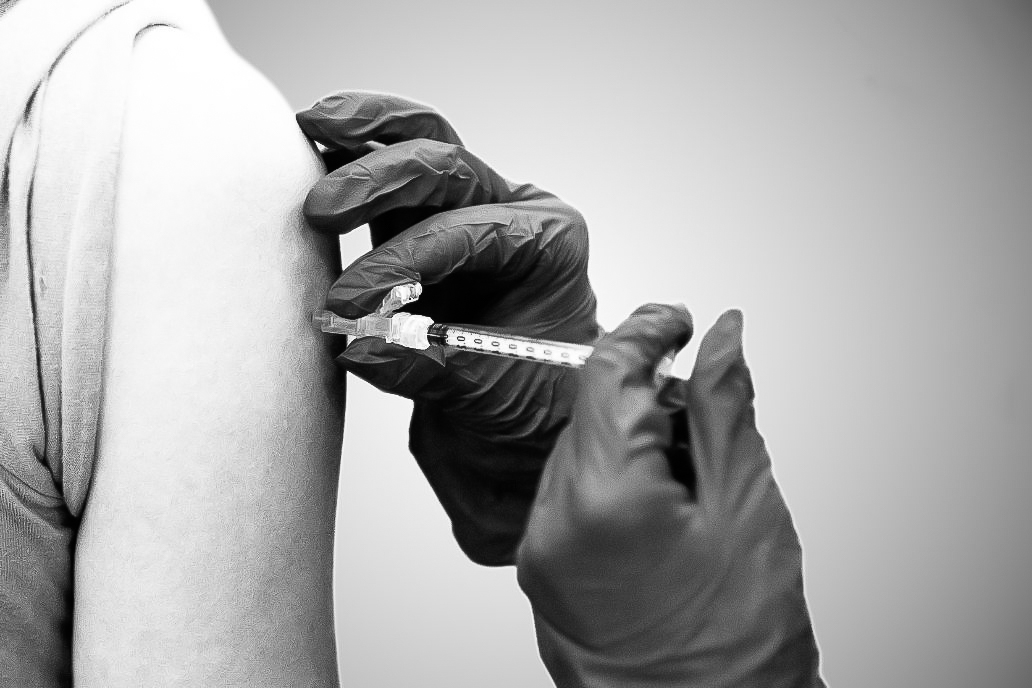
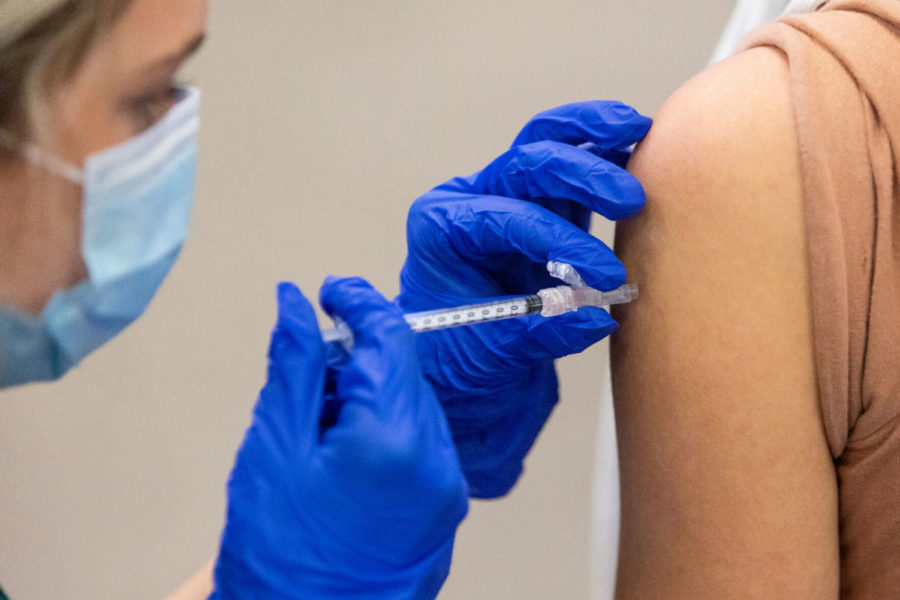
A dose of the Pfizer COVID-19 vaccine is injected into a patient’s arm on Saturday, April 10, 2021, at UK’s COVID-19 vaccination clinic at Kroger Field in Lexington, Kentucky. Photo by Jack Weaver | Staff
September 7, 2022
The Centers for Disease Control and Prevention (CDC) have authorized a new COVID-19 booster, meaning the UK-provided boosters are no longer Food and Drug Administration (FDA) approved.
According to the FDA, the new boosters, referred to as “bivalent” vaccines, contain components from both the original COVID-19 strain and the Omicron variant, as opposed to the older “monovalent” versions which only have the original virus.
In a campus-wide email, UK president Eli Capilouto said the vaccination clinic located in the Gatton Student Center will be closed in preparation for the new vaccine. Boosters will now operate on an appointment-only basis. Any booster appointments scheduled through UK facilities will need to be rescheduled.
Although UK no longer has a mandatory testing policy, it is encouraged for all students who feel sick to get COVID-19 tested and isolate until a negative result is given. All students who isolate because of a positive result will have their absences excused.
As of Sept. 6, there were 875 new positive cases in Lexington, according to the Lexington-Fayette County Health Department website. This brings the total case number to 112,453.
In the email, Capilouto said the UK administration will continue to monitor updates related to COVID-19 and that getting vaccinated “remains our best defense against the virus.”
Additional testing information, in addition to UK pharmacy locations, general CDC information and Health Corps information can be found linked in the email.