Kentucky researchers and the race for COVID-19 vaccines
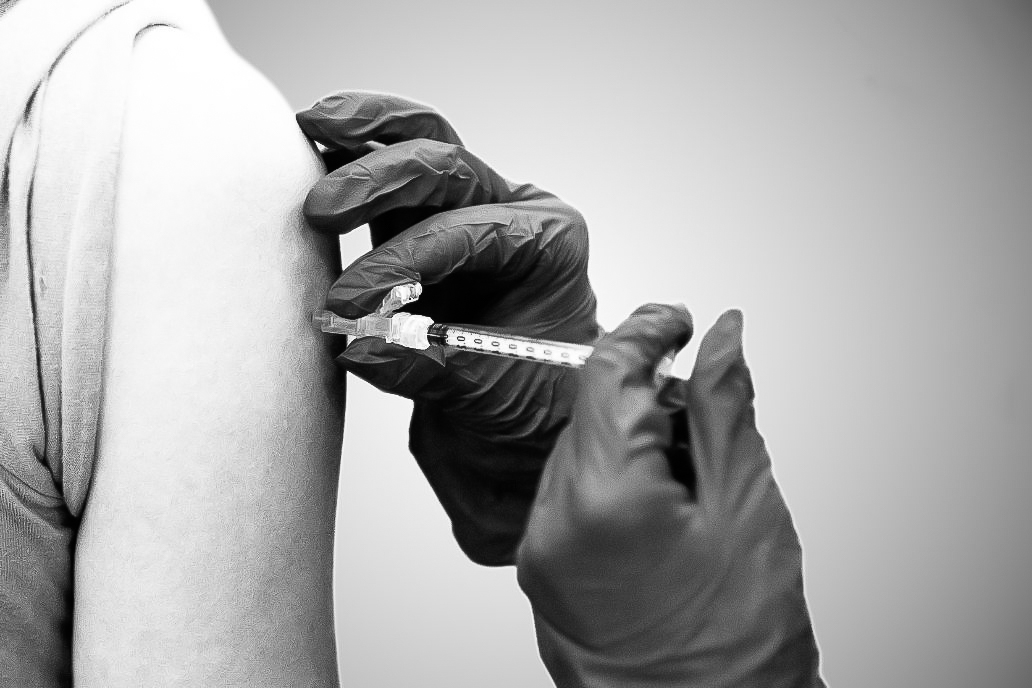
A researcher preps a syringe with a COVID-19 vaccine as part of the Johnson & Johnson trial. Photo provided by ENSEMBLE research group.
March 11, 2021
Some people called it a scientific miracle. But the development of three vaccines against a new coronavirus in less than a year is just that – science. The incredible effort behind these successful vaccines was helped along by researchers in Lexington, including at the University of Kentucky.
As a research university, many of the UK’s faculty applied their knowledge to aid the fight against this new disease. But the Center for Clinical and Translational Science in particular has contributed to tackling the pandemic by becoming the lead for three local sites of trials for Janssen COVID-19 vaccine.
Originating from the pharmaceutical companies of Johnson and Johnson, this vaccine is a single dose shot that doesn’t have the same cold storage requirements as other vaccines. The partnership with Baptist Health Lexington and Norton and University of Kentucky was the largest enrolled site in the world with 890 local volunteers.
Results from the study, which included 43,000 people globally, showed that the Janssen vaccine provided complete protection from hospitalizations and death. The vaccine averaged 86% effectiveness against severe illness and had an overall efficacy rate of 72% in the United States and 64% in South Africa.
UK’s trial first began seeking volunteers in November of 2020. UK’s Dr. Richard Greenberg was the principal investigator, along with Dr. David Dougherty of Baptist Health Lexington and Dr. Paul Schulz at Norton Healthcare and Mobile. The trio participated in a press conference on Friday, Feb. 26 concerning the interim results of the trial.
The Janssen vaccine was under review by the FDA at the time to see if it met the requirements for emergency use authorization. Greenberg, who has more than 40 years of experience in infectious diseases, was optimistic of the possible approval of the Janssen vaccine.
“It has added meaning to all of us because if the approval is granted, we’ve put a tremendous amount of effort and been very successful in gathering the data to have this happen,” said Greenberg.
The Johnson and Johnson vaccine recieved emergency authorization the following day, kicking off distribution across the United States. Kentucky expected to get 30,000 doses last week. Researchers said the Janssen vaccine was “more tolerable” than other vaccines, with trial participants reporting less side effects after dose administration.
“In our trial, at least, there were zero cases of anaphylaxis. Only about 9% of folks got fever with only a very low percentage of those getting severe fever. So it is a very, very safe vaccine and all three vaccines, we’re lucky to have all three vaccines at the stage of the pandemic,” Dougherty said. No deaths were documented in the overall trial.
Unlike the Moderna and Pfizer vaccines, the Janssen vaccine was studied in countries like South Africa and Brazil, where COVID-19 variants are already circulating, variants that may be more contagious and fatal. Greenberg said that the Janssen vaccine having trials in those countries may be why its efficacy rate is lower than other vaccines.
“The efficacy is difficult to compare because the timeline and the COVID rates in all the countries where this study took place, they went up over that timeframe of the Johnson and Johnson study and put a lot of pressure on the vaccine,” Dougherty said. “I think the most important point is that there were zero hospitalizations and zero deaths in the vaccine arm, including in South Africa and Brazil.”
Greenberg said that 95% of the isolates from South Africa studied in the Janssen were of the variants, so the data shows the vaccine does have activity against the variants.
“And it also did well in Brazil, where there’s another variant circulating. The vaccines are slightly different, apparently. And the response to the different strains may be different,” Greenberg said. He added that all three vaccines are good vaccines and that people should get one when they are eligible.
Though the Johnson and Johnson vaccine is less effective than other vaccines, a single dose COVID-19 vaccine could accomplish vaccinations at a far quicker rate than the two-dose vaccines currently available.
“So from impact perspective, then every dose is granted to you, every dose that’s manufactured and distributed, is a person vaccinated rather than two doses per person. So, I think that’s really important to keep in mind as well,” Dougherty said. As the press conference was being held, Greenberg said Johnson and Johnson had trucks delivering doses to CDC stockpiles in preparation for approval. The company hopes to have 100 million doses by summer.
Another benefit of the Johnson & Johnson vaccine is that it is easier to transport, not requiring deep cold storage.
“It would just make vaccines more accessible to the rural areas, as well as something mobile to get into areas like the nursing homes where people can’t get out and get vaccinated, the vaccine has to go to them,” Greenberg said. That accessibility could shift the global vaccine rollout, which is necessary to combat a pandemic.
“If we don’t vaccinate the world, we’re going to continue to have variants developed around the world and to seep into the U.S. borders,” Dougherty said.
With the hopes of the Janssen vaccine to be successful, the research group has already sought new goals for the future of Kentucky and those around it.
“I’d like to also mention that coming up, we expect to be doing pediatric versions of these studies. And we’re also trying to plan for a study on campus with one of the vaccines,” said Greenberg. “So this has really led us down a great path and given us national notoriety. And it gives us some very exciting projects coming up, that will make a difference.”
Greenberg and the CCTS team will enroll up to 200 Lexington adults for a trial investigating whether two shots of the Janssen vaccine is more effective than one.